How Does the Temperature Affect the Phase of Water
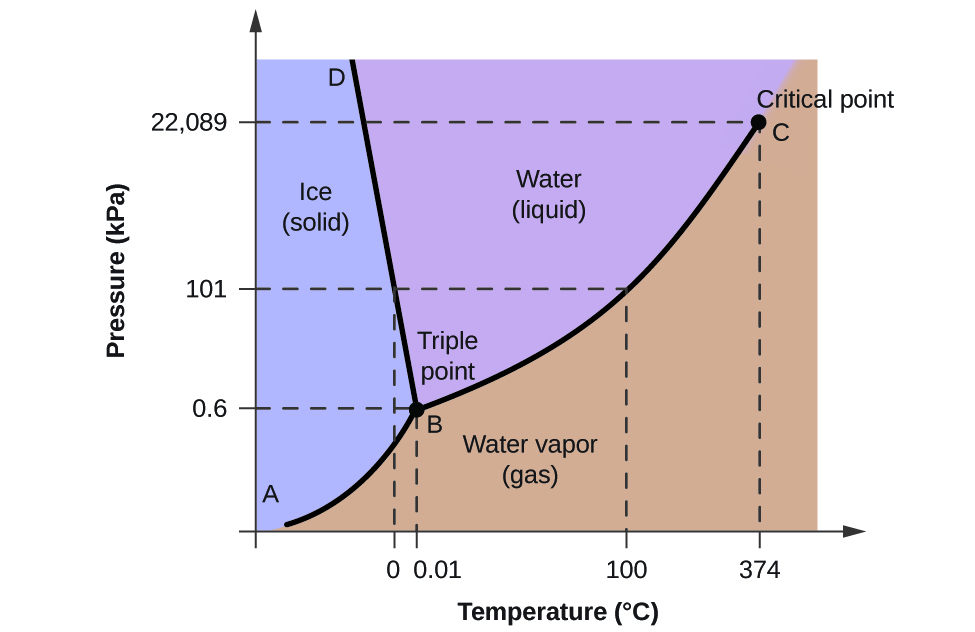
Paper chromatography and thin-layer chromatography TLC has two. Predict the phase of water at the six temperatures given below.

Phase Change And Latent Heat Boundless Physics
Then fill in the Actual phase row using the Gizmo.
. The Temperature T affects the mass Density D of pure water since D decreaces slowly with an increase in Temperature T eg. How does the temperature of water affect the speed of dissolving. Also the optimum pH for the coagulation process decreases as temperature increases.
The phase velocity of a gravitycapillary wave on a fluid interface is influenced by the effects of gravity surface tension density and by fluid inertia. When water is heated the molecules gain energy and thus move faster. Sugar dissolves faster in hot water than it does in cold water because hot water has more energy than cold water.
Yes the pH is inversely proportional to the temperature of the solution. I nstead the additional thermal energy acts to loosen bonds between molecules or atoms and causes a phase change. How does temperature affect the phase of water.
As a result the gas molecules dissolved in the liquid are more likely to escape to the gas phase. Likewise as heat is added to a liquid its temperature increases as the molecules once again move faster. Or you can do opposite of it.
Since surface tension and density are affected by temperature the phase velocity begins to become slightly affected by changes in temperature. Kinetic energy while being the reason phase changes are constant Kinetic Energy can be caused by other means. The angle of Refraction R sinR1 also in water increases23 slowly with an increase in Temperature T eg.
When the temperature of a solution rises the molecular vibrations in the solution rise resulting in the ionization and formation of H ions. At this point the phase change added heat goes into changing the state from a solid to liquid. When we consider this property of water having less density when in solid state ice than liquid water which has a maximum density at a temperature of 4 0 c we see that ice tends to float on water.
It will change its state. Furthermore as temperature decreases the viscosity of water increases and the rate of sedimentation decreases. List your predictions in the Predicted phase row of the table.
In the gas or vapor phase we have a mixture of air and water vapor. Similarly if we heat a volume of water above 100 degrees Celsius or 212 degrees Fahrenheit water changes its phase into a gas called water vapor. Owing to temperature changes the pH value of the solution changes.
As they move faster they come into contact with the sugar more often causing it to dissolve faster. In the case of water the three main phases are called. More H ions lead to more acidic behavior.
In summer and fall when the water temperature is high the dissolved-oxygen concentration is often lower. Why does solubility of sugar increase with temperature. Temperature can affect the separation of components in all chromatography types.
When water is cooled to form ice the hydrogen bonds become more rigid but also more open causing water to expand thus increasing in volume. Temperature -10 C 10 C 50 C 90 C 110 C 120 C Predicted phase solid liquid or gas Actual phase solid liquid or gas 4. The efficiency of one of the key water treatment steps coagulation is greatly dependent on temperature.
Answer 1 of 2. At 100C a pH value of 614 is the new neutral point on the pH scale at this higher temperature. Only when this phase change is complete the temperature can increase.
Temperature affects phase change by slowing down the movement in between the atoms thus causing a change in kinetic energy which in turn causes the atoms to undergo forms of combining or a type of disepersion. During the phase change when solid melts into liquid its temperature remains constant as the heat energy is stored as potential energy. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
How does the temperature affect the phases changes of matter. Temperature affects the phase of water by changing the connection between the molecules. This happens because as temperature increases the average kinetic energy of the gas molecules increases.
Cold water can hold more dissolved oxygen than warm water. PH decreases with increase in temperature. As heat is added to solid water the temperature increases until it reaches 0 C the melting point.
Because this energy enters or leaves a system during a phase change without causing a temperature change in the system it is known as latent heat latent means hidden. The large size of the phase change energy indicates that there is a large amount of potential energy associated with the intermolecular forces between the water. Ice for solid water liquid water or just water for the liquid phase and water vapor for the gaseous phase.
Temperature affects phase change by slowing down the movement in between the atoms thus causing a change in kinetic energy which in turn causes the atoms to undergo forms of combining or a. But if we lower the temperature below 0 degrees Celsius or 32 degrees Fahrenheit water changes its phase into a solid called ice. I am assuming that the temperature of the air and water are the same.
In winter and early spring when the water temperature is low the dissolved oxygen concentration is high. The constancy of temperature when water is going through its phase changes at 0C and 100C provides some insight into the nature of the internal energy of water ice and steam and into the nature of temperature. Heating the solid water you can get liquid water and heating the liquid water you will get gas.
It is the temperature of the water that is important because that determines the vapor pressurewhich I will explain in a moment--of the water. If the temperature rises the heat transfers further energy to the solvent-giving the molecule the power to escape from the surface of the liquid hence increases the transfer of liquid to the vapor phase. In the case of pure water there are always the same concentration of hydrogen ions and hydroxide ions and hence the water is still neutral even if its pH changes.
Higher temperatures may reduce the availability of drinking water by provoking the loss of mountain glaciers and mountain snowpack and causing earlier spring snowmeltsall of which reduces the amount of available water in streams rivers and other bodies of water. For example sugar and salt are more soluble in water at higher temperatures. For many solids dissolved in liquid water the solubility increases with temperature.
How does thermal energy effect the phases changes of water. How does the phase of water affect its specific heat capacity.


No comments for "How Does the Temperature Affect the Phase of Water"
Post a Comment